1.¶
Exam I Review: Practice Set¶
- How many significant figures are in 0. 000456?
a. 7
b. 4
c. 3
d. 6
- How many significant figures are in $4.5600 \times 10^{20}$?
a. 5
b. 3
c. 17
d. 21
- The correct molecular formula for lead nitrate is:
a. $PbN_{3}$
b. $Pb(NO_{2})_{3}$
c. $Pb(NO_{3})_{2}$
d. $PNO_{3}$
- The correct molecular formula for ammonium chloride is:
a. $NH_{3}Cl$
b. $AmCl$
c. $NH_{4}Cl$
d. $AmCl_{2}$
- The correct molecular formula for iron(II)bromide is:
a. $FeBr_{3}$
b. $FeBr_{2}$
c. $I_{2}Br_{2}$
d. $IBr_{3}$
- The correct molecular formula of dinitrogen pentoxide is:
a. $N_{2}O_{5}$
b. $(NO_{5})_{2}$
c. $2NO_{5}$
d. $N_{2}P_{5}O$
- The systematic (IUPAC) name of $NaClO_{4}$ is:
a. sodium perchlorate
b. sodium chlorate
c. sodium hypochlorate
d. sodium chloride tetraoxide
- How many electrons are present in a $^{40}Ca^{2+}$ species?
a. 40
b. 38
c. 20
d. 18
- If an ion contains 33 protons, 39 neutrons, and 34 electrons, the ion is:
a. $^{73}Se^{1−}$
b. $^{77}2As^{1−}$
c. $^{76}7Y^{1+}$
d. $^{73}Se^{1+}$
- The average mass of an atom is determined by
a. taking a weighted average of all isotopic masses
b. averaging the masses of each isotope
c. taking a weighted average of all stable isotopic masses
d. adding the isotopic masses and dividing by the number of isotopes
- Given that: $AgNO_{3}(aq) + KCl(aq) \rightarrow AgCl(s) + KNO_{3}(aq)$, which of the following species is classified as a spectator ion?
a. $Ag^{1+}(aq)$
b. ${NO_{3}}^{1−}(aq)$
c. $AgCl(s)$
d. $Cl^{1−}(aq)$
- Which of the following equations best illustrate the net ionic equation for the reaction of radium chloride with potassium sulfate?
a. $RaCl_{2} + K_{2}SO_{4} \rightarrow RaSO4 + 2 KCl$
b. $Ra^{2+}(aq) + 2 Cl^{1−}(aq) + 2 K^{1+}(aq) + {SO_{4}}^{2−}(aq) \rightarrow RaSO_{4}(s) + 2 Cl^{1−}(aq) + 2 K^{1+}(aq)$
c. $Ra^{2+}(aq) + {SO_{4}}^{2−}(aq)\rightarrow RaSO_{4}(s)$
d. $Ra^{2+}(aq) + 2 Cl^{1−}(aq) + 2 K^{1+}(aq) + {SO_{4}}^{2−}(aq) \rightarrow RaSO_{4}(s)+ 2 KCl(aq)$
- Which of the following may be classified as an example of an acid-base reaction?
a. $HCl + NaOH \rightarrow H_{2}O + NaCl$
b. $Ca(NO_{3})_{2} + H_{2}SO_{4} \rightarrow CaSO_{4} + HNO_{3}$
c. $KClO_{4} + NaF \rightarrow NaClO_{4} + KF$
d. $HCOOH + NH_{4}Cl \rightarrow NH_{4}COOH + HCl$
- How many atoms are contained in $5.99 × 10^{−14}$ moles of glucose ($C_{6}H_{12}O_{6}$)?
a. $2.42 × 10^{38}$
b. $8.66 × 10^{11}$
c. $1.84 × 10^{21}$
d. $3.61 × 10^{10}$
- An unknown hydrocarbon is subjected to elemental analysis. The results of this test show that the compound is 83.24% carbon and 16.76% H. With a molar mass of 72 g/mole, the molecular formula of this compound is:
a. $C_{4}H_{10}$ b. $C_{5}H_{10}$ c. $C_{5}H_{12}$ d. $C_{6}H_{14}$
- For the following reaction, determine the value of “x.” 3.2 mol $S_{8}$ yield x mol $CS_{2}$ $$4C + S_{8} \rightarrow 4CS_{2}$$
a. 3.2
b. 12.8
c. 51.2
d. 13
- In petroleum refining, hydrocarbons are often manipulated by reacting them with $H_{2}(g)$. If cyclohexane, $C_{6}H_{12}$, is reacted with hydrogen to form hexane, $C_{6}H_{14}$, how many moles of hydrogen are needed to react with 453 moles of hexane?
a. 6342
b. 3171
c. 2
d. 453
Answers to practice exams.¶
Jump to table of contents.¶
2.¶
Exam II Review: Practice Set¶
(1) Given that: CH₄ + 2O₂ → CO₂ + 2H₂O, how many moles of carbon dioxide are produced in the complete combustion of 100.0 grams of methane (CH₄)?
a. 6.25
b. 12.5
c. 200
d. 0.321
(2) Limestone (CaCO₃) emits CO₂ gas when exposed to HCl. What is the theoretical yield of CO₂ if 9.45 grams of limestone is completely dissolved in HCl? CaCO₃(s) + 2 HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
a. 21.5 g
b. 4.16 g
c. 9.53 g
d. 24.7 g
(3) Copper reacts with nitric acid via the following equation.
3 Cu(s) + 8 HNO3(aq) → 3 Cu(NO3)2 + 2 NO + 4 H2O(l)
What mass of NO(g) can be formed when 10.0 g of Cu is reacted with 115 g HNO3?
a. 11.98
b. 4.76
c. 9.52
d. 3.15
(4) A 25.00 mL sample of vinegar is titrated to neutralization with 22.64 mL of 2.00 M NaOH. What is the molarity of the vinegar?
a. 1.66
b. 2.21
c. 2.83
d. 1.81
(5) An ethanol plant begins a production run with sufficient reactants to predict a theoretical yield of 1250 metric tons of ethanol. The process produces 1178.6 metric tons. What is the percent yield for this production run?
a. 94.29%
b. 82.10%
c. 106.1%
d. 99.23%
(6) What volume of 0.812 M HCl, in milliliters, is required to titrate 1.45 g of NaOH to the equivalence point?
NaOH(aq) + HCl(aq) : H2O(l) + NaCl(aq)
a. 44.6 mL
b. 0.014 L
c. 0.036 L
d. 22.4 mL
(7) Consider the following reaction: 6 NiO(s) + 4 ClF3(g) → 6 NiF2(s) + 2 Cl2(g) + 3 O2(g)
What mass of NiO will react with a sample of ClF3 gas that has a pressure of 250 torr in a 2.5-L flask at 20°C?
a. 5.69 g
b. 22.79 g
c. 0.63 kg
d. 3.8 g
(8) Assuming a constant temperature, the volume of a gas will __ with an increase in pressure.
a. increase
b. remain the same
c. decrease
d. fluctuate about the original mean
(9) What is the partial pressure of hydrogen in a 100.0 L vessel at 10.0°C holding 11.6 g H₂, 6.8 g Ar, and 5.3 g of He?
a. 1.6 atm
b. 1.3 atm
c. 2.4 atm
d. 5.7 atm
(10) Select all of the following postulates of the kinetic theory of gases.
a. Particles in a gas are infinitely small; they occupy no volume.
b. The average kinetic energy of the particles in a gas is proportional to the absolute temperature of the gas and does not depend on the identity of the gas.
c. Particles undergo inelastic collisions
d. A gas is made up of a vast number of particles, and these
e. The average kinetic energy of the particles in a gas is proportional to the absolute temperature of the gas and does not depend on the identity of the gas.
(11) Place these gases in order of increasing average molecular seed at 25°C: $Kr, CH_{4}, N_{2}, CH_{2}Cl_{2}$.
a. $CH_{4}, N_{2}, CH_{2}Cl_{2}, Kr$
b. $Kr, CH_{2}Cl_{2}, N_{2}, CH_{4}$
c. $CH_{2}Cl_{2}, Kr, N_{2}, CH_{4}$
d. $CH_{4}, N_{2}, Kr, CH_{2}Cl_{2}$
(12) The figure below shows two distribution of speeds. Select the plausible statement(s) that describe the figure.
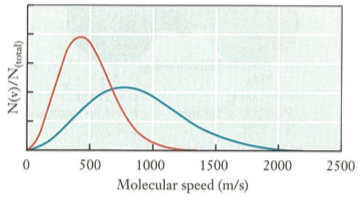
a. Both distributions of Ar gas — red curve at 300 K and blue curve at 300 K
b. Both distributions of Ar gas — red curve at 300 K and blue curve at 1000 K
c. Both distributions of Ar gas — red curve at 1000 K and blue curve at 300 K
d. Red curve — distribution of Ar gas at 0 K. Blue curve — distribution of $N_{2}$ gas at 0 K
e. Red curve — distribution of Ar gas at 300 K. Blue curve — distribution of $N_{2}$ gas at 300 K
f. Red curve — distribution of $N_{2}$ gas at 300 K. Blue curve — distribution of Ar gas at 300 K
(13) Analysis of a gaseous chlorofluorocarbon, CClxFy, shows that it contains 11.79% C and 69.57% Cl. In another experiment, you find that 0.107 g of the compound fills a 458-mL flask at 25°C with a pressure of 21.3 mm Hg. What is the molecular formula of the compound?
a. C4Cl3F2
b. C2Cl4F2
c. C3Cl4F1
d. C3Cl4F2
(14) As the temperature of a gas increases, the area under the Maxwell-Boltzmann distribution of molecular speeds curve:
a. increases
b. remains the same
c. decreases
d. multiplies by a constant
(15) Select which of the following represent valid sets of quantum numbers.
a. n = 3, l = 3, m = 0
b. n = 2, l = 1, m = 0
c. n = 6, l = 5, m = –1
d. n = 4, l = 3, m = –4
(16) Which is not a legitimate value for the "n" quantum number?
a. 0
b. 1
c. 2
d. 3
(17) For a photon with the following energy, calculate the wavelength and identify what region of the spectrum. 8.7 × 10–22 J
a. 1.15 × 10–8 m ; UV
b. 16.4 × 10–10 m ; microwave
c. 16.4 × 101 m ; radio
d. 2.3 × 10–4 m ; visible
e. 2.3 × 10–4 m ; microwave
(18) The size of orbitals tends to decrease for large nuclei as a result of:
a. electron crowding
b. proton spin
c. uncoupling leptons
d. nuclear shielding
(19) What is the correct electron configuration of copper?
a. 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰
b. 1s²2s²2p⁶3s¹3p⁶4s²3d⁹
c. 1s²2s²2p⁶3s²3p⁶3d¹⁰4s¹
d. 1s²2s²2p⁶3s²3p⁶3d⁹4s²
(20) Of the following elements, which displays the greatest electron affinity?
a. Se
b. Ca
c. C
d. F
3.¶
Exam III Review: Practice Set¶
(1-7) Draw the lewis dot structures for the following:
(1) $XeF_{4}$
(2) $PCl_{3}$
(3) $SF_{4}$
(4) $O_{3}$
(5) $CS_{2}$
(6) $NH_{3}$
(7) $CH3COOH$
(8) From questions (1-7), which of the compounds is an ion
a. 1,2,3,4,5,6,7
b. 2 and 4
c. 7
d. None
(9) Predict the geometry of the following species: SO2, BeCl2, SeCl4, PCl5
a. bent, linear, tetrahedral, trigonal bipyramidal b. linear, bent, see-saw, trigonal bipyramidal c. bent, linear, see-saw, trigonal bipyramidal d. bent, linear, tetrahedral, trigonal bipyramidal
(10) Which of the following contains both ionic and covalent bonds in the same compound?
a. BaCO3
b. MgCl2
c. BaO
d. H2S
e. SO
(11) Which of the following yields the highest boiling point?
a. HF
b. $CH_{3}COOH$
c. C8H18
d. $CH_{3}OH$
(12) Which of the following intermolecular forces is the strongest?
a. Dispersion forces
b. London forces
c. Dipole–Dipole interactions
d. Hydrogen bonding
(13) According to the valence shell electron pair repulsion theory, which molecule shape best describes the geometry of ammonia?
a. Octahedral
b. Square planar
c. Trigonal planar
d. Trigonal pyramidal
(14) Which of the following usually has the smallest band gap?
a. insulators
b. p-type semiconductors
c. conductors
d. n-type semiconductors
(15) Draw the Lewis dot structures of the following compounds and identify the number of pi bonds in each: Cl2O, H2CCH2, HCCCN, SiO2.
a. 0, 1, 4, 2
b. 1, 2, 4, 2
c. 1, 0, 4, 1
d. 0, 1, 4, 1
(16) Which substance has the lowest vapor pressure?
a. $CClF_{3}$
b. propane (C3H8)
c. ethane (C2H6)
d. HCN
(17) Which of the followings statements is true of dipole–dipole interactions?
a. They are based on transient dipoles.
b. They are stronger than dispersion forces.
c. They are also referred to as instantaneous dipole–induced dipole forces.
d. They are also referred to as London forces.
(18) A p-type material can be made if pure germanium is doped with _.
a. gallium
b. carbon
c. arsenic
d. silicon
(19) Draw the heat curve for water starting from -50°C to 200°C and label the equation that represents each segment of the curve.
(20) Suppose you have steam at 167°C that is bubbling in liquid water at 25°C to quickly heat it up. Calculate how much steam is needed to bring 1.24 kg of water to a boil.
(21) The heat of fusion of pure silicon is 43.4 kJ/mol. How much energy would be needed to melt a 5.24-g sample of silicon at its melting point of 1693 K?
a. 40.10 kJ
b. 80.10 kJ
c. 8.10 kJ
d. 8.10 kJ
(22) Calculate the energy required to convert 1.70 g of ice originally at –12.0°C into steam at 105°C.
a. 2210 kJ
b. 5180 kJ
c. 2.210 kJ
d. 5.180 kJ
Part 1. Chapter 11:¶
Catalyst¶
(1) Lowering the activation energy of a reaction will increase the rate of the reaction by:
a. removing potential energy from the reactants.
b. spontaneously increasing the quantity of the reactants.
c. packing the created products in a favored arrangement.
d. providing a new reaction mechanism.
(4) Which of the following statements is most correct:
(a) A catalyst speeds up the reaction by lowering the activation energy.
(b) A catalyst speeds up the reaction by lowering the activation energy of the reaction path.
(c) A catalyst speeds up the reaction by altering course along a new pathway
(d) A catalyst speeds up the reaction by having lower activation energy along a new reaction pathway.
(5) Reaction order is determined __.
(a) by the number of reactant molecules
(b) from the number of distinct species produced
(c) by experiment
(d) using tabulated values
(6) Identify all the statements that are true:
(a) The rate of a reaction depends on the concentration of the reactants.
(b) The rate of a reaction may be expressed as the disappearance of reactants with respect to time.
(c) The rate constant "k" will vary with changes in concentration of first order reactants.
(d) For a first-order reaction, t1/2 = ln 2/ k.
(e) For a first-order reaction, a plot of concentration versus time provides a line with a slope equal to −k.
(f) The units for the first order rate constant are M s-1.
(g) The instantaneous rate of a reaction is determined by the slope of a line tangent to the curve defined by the change in concentration versus time.
(h) For a zero-order reaction, a plot of a reactant concentration versus time is linear.
(7) Identify all the statements that are true:
(a) The units for the zeroth order rate constant are M s-1.
(b) The units for the first order rate constant are M-1 s-1.
(c) The units for the second order rate constant are M-1 s-1.
(c) Endothermic reactions do not require activation energy.
(d) Stirring a reaction increases the rate of the reaction by raising the activation energy.
(e) A termolecular step in a reaction mechanism is a step that involves two molecules.
(f) For a zeroth-order reaction, t1/2 = [x]0 / 2k.
(g) A reaction mechanism is a collection of elementary steps.
(h) In zero-order reactions, the rate of reaction does not change as the reactants are consumed.
(8) Identify all the statements that are true:
(a) For an elementary step, the order of the reaction is equal to the molecularity of the reaction.
(b) For an elementary step, the order of the reaction is equal to the sum of the stoichiometric coefficients for the reaction.
(c) The order of an overall reaction is equal to the molecularity of the reaction.
(d) The molecularity of an overall reaction is equal to the sum of the stoichiometric coefficients for the reaction.
(e) The order of the reaction can be determined from the units of the rate constant for the reaction.
(9) Consider the following reaction: 2A + B → C. A kinetics study on this reaction yielded the following data:
| [A] mol/L | [B] mol/L | Rate = mol/L/s |
|---|---|---|
| 0.0450 | 0.0250 | 5.03 × 10−3 |
| 0.0450 | 0.0500 | 2.01 × 10−2 |
| 0.0900 | 0.0250 | 5.03 × 10−3 |
What is the order of the reaction with respect to ([A], [B])?
a. 1, 2
b. 2, 1
c. 2, 0
d. 0, 2
(10) What is the rate law for each of the following elementary reaction: O(g) + O3(g) → 2 O2(g)
(a) Rate = -k [O][O3]
(b) Rate = k [O][O3]
(c) Rate = -k [O]2[O3]
(d) Rate = k [O]2[O3]
(11) What is the rate law for each of the following elementary reaction: 2 NO2(g) → N2O4(g)
(a) Rate = k [NO2]
(b) Rate = -k [NO2]
(c) Rate = k [NO2]2
(d) Rate = -k [NO2]2
(12) If a reaction is zero order with respect to [X], doubling the concentration of [X] will:
a. triple the rate of the reaction.
b. result in a fourfold increase in the rate of the reaction.
c. result in a ninefold increase in the rate of the reaction.
d. not affect the rate of the reaction.
(13) If a reaction is second order with respect to [X], doubling the concentration of [X] will:
a. double the rate of the reaction.
b. increase the rate of the reaction fourfold.
c. increase the rate of the reaction eightfold.
d. not affect the rate of the reaction.
(14) According to the differential rate law: rate = k[X]m[Y]n. If m = 2 and n = 1, the overall reaction order is:
a. 3
b. 2
c. 1
d. 0
(15) Consider the following reaction for production of ammonia via the Haber process:
$$3H_{2} + N_{2} \longrightarrow 2NH_{3}$$Which of the following expressions best illustrates the rate of reaction?
a. rate = 2Δ[NH3] / Δt
b. rate = Δ[NH3] / 2Δt
c. rate = 2Δ[NH3] / 2Δt
d. There is too little information to assess the change.
(16) The reaction for the Haber process, the industrial production of ammonia, is
N2(g) + 3 H2(g) → 2 NH3(g)
Assume that under certain laboratory conditions ammonia is produced at the rate of 6.29 × 10–5 mol L–1 s–1. At what rate is nitrogen consumed? At what rate is hydrogen consumed?
(17) Rate data were obtained at 25°C for the following reaction. What is the rate law expression for this reaction?
$$A + 2 B \longrightarrow C + 2 D$$| Expt. | Initial [A] (mol L–1) |
Initial [B] (mol L–1) |
|
| 1 | 0.10 | 0.10 | 3.0 × 10–4 |
| 2 | 0.30 | 0.30 | 9.0 × 10–4 |
| 3 | 0.10 | 0.30 | 3.0 × 10–4 |
| 4 | 0.20 | 0.40 | 6.0 × 10–4 |
(18) For a zero-order reaction, the plot of concentration with respect to time is _.
a. linear; m = −k
b. hyperbolic
c. parabolic
d. linear; m = 0
(19) Raising the temperature of a reaction increases the rate of the reaction by:
a. increasing the activation energy of the reaction.
b. creating more molecules in the reaction.
c. increasing the number of molecules moving at a speed sufficiently high enough to produce a reactive collision.
d. decreasing the entropy of the system.
(20) The higher the Ksp of a salt, the less soluble the salt. True (a) or False (b)?
(21) In the context of equilibrium constants of chemical reactions, which "K" value indicates a reaction that favors the formation of products the most?
a. K = 5.31 × 103
b. K = 4.99 × 106
c. K = 8.2 × 10−3
d. K = 1.7 × 10−6
(22) What change in reaction direction occurs if dilute HCl is added to a H2PO4− solution?
$$H_{2}{PO_{4}}^{−} + H_{2}O \leftrightharpoons {HPO_{4}}^{2−}+ {H_{3}O}^{+}$$a. The reaction shifts to the right.
b. The reaction shifts to the left.
c. There is no change in the reaction.
d. There is insufficient information to solve this problem.
(23) What is the molar solubility of CaF2 (Ksp = 3.9 × 10−11)?
a. 6.24 × 10−6 M b. 4.41 × 10−6 M
c. 2.14 × 10−4 M d. 9.27 × 10−5 M
(24) In each of the reactions, how does the equilibrium respond to an increase in pressure?
(a) 2 SO2(g) + O2(g) 2 SO3(g)
(b) H2(g) + I2(g) 2 HI(g)
(c) 3 H2(g) + N2(g) 2 NH3(g)
a. a) shifts to the right, b) shifts to the right, c) shifts to the right
b. a) shifts to the left, b) there is no shift, c) shifts to the right
c. a) there is no shift, b) there is no shift, c) shifts to the right
d. a) shifts to the right, b) there is no shift, c) shifts to the right
(25) Predict the change in the reaction quotient, Q, for each disturbance below, and use that prediction to explain how the equilibrium is shifted by the stress.
(a) NaHCO3 is added to the lake.
(b) H2CO3 is added.
(c) NaOH is added.
a. a) shift to the left, b) shift to the left, c) shift to the left
b. a) shift to the right, b) shift to the right, c) shift to the left
c. a) shift to the right, b) there is no shift, c) shift to the left
d. a) shift to the right, b) shift to the left, c) shift to the left
(26) The equilibrium constant for the reaction, 3 H2(g) + N2(g) 2 NH3(g), at a given temperature is 1.4 × 10–7. Calculate the equilibrium concentration of ammonia, if [H2] = 1.2 × 10–2 mol L–1 and [N2] = 3.2 × 10–3 mol L–1.
(27) Hydrogen gas and iodine gas react via the following equation:
H2(g) + I2(g) 2 HI(g) Kc = 76 (at 600 K)
If 0.050 mol HI is placed in an empty 1.0-L flask at 600 K, what are the equilibrium concentrations of HI, I2, and H2?
5.¶
Final Exam: Practice Set¶
Electrochmistry¶
(1) In a galvanic cell, reduction occurs at _. a. the cathode
b. a salt bridge c. the anode
d. the external circuit
(2) Which of the following reactions are spontaneous at standard conditions?
(a) Zn(s) + 2 Fe3+(aq) → Zn2+(aq) + 2 Fe2+(aq)
(b) Cu(s) + 2 H+(aq) → Cu2+(aq) + H2(g)
(c) 2 Br–(aq) + I2(s) → Br2(l) + 2 I–(aq)
a. (a),(b),(c)
b. (a),(b)
c. (b),(c)
d. (a)
(3) Using values from the tables of standard reduction potentials, calculate the cell potentials of the following cells:
Pt(s) | Fe2+(aq), Fe3+(aq) ║ MnO4–(aq), H+(aq), Mn2+(aq) | Pt(s)
a. 0.844 V
b. 0.736 V
c. -0.335 V
d. 0.335 V
(4) The most common fuel cells are based on the reaction of _.
a. nickel and oxygen to produce nickel oxide
b. hydrogen and oxygen to produce water c. lead and sulfuric acid to produce lead sulfate
d. zinc and air to produce zinc oxide
(5) Which of the following cells is most likely to be an example of a galvanic cell?
a. Zn(s) | Zn2+ || Cu2+ | Cu(s)
b. Cu(s) | Cu2+ || Fe2+ | Fe(s)
c. Au(s) | Au+ || Ag+ | Ag(s)
d. Sn(s) | Sn2+ || Fe2+ | Fe(s)
(6) Balance the following electrochemical reaction in acid:
MnO4−(aq) + Zr(s) ↔ Mn2+(aq) + Zr2+(aq)
a. 2 MnO4−(aq) + 16 H+(aq) + 5 Zr(s) → 2 Mn2+(aq) + 5 Zr2+(aq) + 8 H2O(l)
b. 2 MnO4−(aq) + 5 Zr(s) → 2 Mn2+(aq) + 5 Zr2+(aq)
c. MnO4−(aq) + Zr(s) → Mn2+(aq) + Zr2+(aq) + 5 e− d. 2 MnO4−(aq) + 8 H+(aq) + 5 Zr(s) → 2 Mn2+(aq) + 5 Zr2+(aq) + 8 H2O(l)
(7) How many grams of silver are deposited at a platinum cathode in the electrolysis of AgNO3(aq) by 5.30 A of electric current in 4.0 hours?
a. 85.3 g
b. 42.6 g c. 121 g
d. 188 g
(8) The following half-cells are available: Ag+(aq, 1.0 M) | Ag(s), Zn2+(aq, 1.0 M) | Zn(s), Cu2+(aq, 1.0 M) | Cu(s), and Co2+(aq, 1.0 M) | Co(s). Linking any two half-cells makes a voltaic cell. Given four different half-cells, six voltaic cells are possible. These are labeled, for simplicity, Ag-Zn, Ag-Cu, Ag-Co, Zn-Cu, Zn-Co, and Cu-Co.
(a) In which of the voltaic cells does the copper electrode serve as the cathode? In which of the voltaic cells does the cobalt electrode serve as the anode?
(b) Which combination of half-cells generates the highest potential? Which combination generates the lowest potential?
(9) Calculate the free energy change for the following cell:
Zn(s) | Zn2+(aq) ║ Cr3+(aq) | Cr(s)
a. 100 kJ/mol
b. 10 kJ/mol
c. –10 kJ/mol
d. -100 kJ/mol
(10) Suppose that you cannot find a table of standard reduction potentials. You remember that the standard reduction potential of Cu2+ + 2 e– → Cu(s) is 0.337 V. Given that ∆Gf°(Cu2+) = 65.49 kJ mol–1 and that ∆Gf°(Ni2+) = –45.6 kJ mol–1, determine the standard reduction potential of Ni | Ni2+ from these data.
a. 0.239 V
b. -1.33 V
c. –0.239 V
d. 1.33 V
Nuclear Chemistry¶
(11) Gold-198 is used in the diagnosis of liver problems. The half-life of 198Au is 2.69 days. If you begin with 2.8 µg of this gold isotope, what mass remains after 10.8 days?
a. 35.0 µg
b. 17.0 µg
c. 0.35 µg
d. 0.17 µg
(12) What is the half-life of a radioisotope if it decays to 12.5% of its radioactivity in 12 years?
a. 1 years
b. 2 years
c. 3 years
d. 4 years
(13) Cobalt-60 is used extensively in medicine as a source of γ-rays. Its half-life is 5.27 years.
(a) How long will it take a Co-60 source to decrease to 18% of its original activity?
a. 0.13 years
b. 13.0 years
c. 1.0 years
d. 10.0 years
(14) Write a nuclear equation for the type of decay Neon-19 is most likely to undergo.
(15)
$$ ^{224}_{88}{Ra} \rightarrow ??? + \hspace{0.2cm} ^{4}_{2}{He}$$(16)
$$ ^{249}_{97}{Bk} \rightarrow \hspace{0.2cm} ^{249}_{98}{???} + \hspace{0.2cm} ^{0}_{-1}{e} $$(17)
$$ ^{209}_{83}{Bi} \hspace{0.2cm}+\hspace{0.2cm} ^{58}_{26}{Fe} \rightarrow \hspace{0.2cm} ^{266}_{109}{\text{Mt}} + \hspace{0.2cm} ??? $$(18)
$$ ??? \hspace{0.2cm}+\hspace{0.2cm} ^{58}_{26}{Fe} \rightarrow \hspace{0.2cm} ^{265}_{108}{\text{Hs}} + \hspace{0.2cm} ^{1}_{0}{n} $$(19)
$$ ^{208}_{82}{Pb} \hspace{0.2cm}+\hspace{0.2cm} ??? \rightarrow \hspace{0.2cm} ^{277}_{112}{\text{Mt}} + \hspace{0.2cm} ^{1}_{0}{n} $$(20)
$$ ^{208}_{82}{Pb} \hspace{0.2cm}+\hspace{0.2cm} ^{62}_{28}{Ni} \rightarrow \hspace{0.2cm} ??? + \hspace{0.2cm} ^{1}_{0}{n} $$Answers to Practice Exams:¶
| # | Exam I | Exam II | Exam III | Exam IV | Final |
|---|---|---|---|---|---|
| 1. | c | a | ans | d | a |
| 2. | a | b | ans | ans | d |
| 3. | c | d | ans | ans | b |
| 4. | c | d | ans | d | b |
| 5. | b | a | ans | c | a |
| 6. | a | a | ans | a,b,d,g,h | a |
| 7. | a | d | ans | a,c,f,g,h | a |
| 8. | d | c | d | a,b,e | ans |
| 9. | b | b | c | d | c |
| 10. | c | a,b,d,e | a | b | c |
| 11. | b | c | b | c | d |
| 12. | c | b,e | d | d | d |
| 13. | a | b | d | b | b |
| 14. | b | b | c | a | ans |
| 15. | c | b,c | a | b | ans |
| 16. | d | a | d | ans | ans |
| 17. | d | e | b | ans | ans |
| 18. | a | d | a | a | ans |
| 19. | b | a | see notes | c | ans |
| 20. | c | d | see notes | b | ans |
| 21. | - | - | d | b | |
| 22. | - | - | d | b | |
| 23. | - | - | - | c | |
| 24. | - | - | - | d | |
| 25. | - | - | - | d | |
| 26. | - | - | - | ans | |
| 27. | - | - | - | ans |
Exam Review I ; Exam Review II ; Exam Review III ; Exam Review IV¶
Jump to table of contents.¶
Work for Exam I Practice Questions: (Requested by Students)¶
- How many atoms are contained in $5.99 × 10^{−14}$ moles of glucose ($C_{6}H_{12}O_{6}$)?
a. $2.42 × 10^{38}$
b. $8.66 × 10^{11}$
c. $1.84 × 10^{21}$
d. $3.61 × 10^{10}$
How many atoms in $C_{6}H_{12}O_{6}$.
6 carbon, 12 hydrogen, 6 oxygen in 1 mol of $C_{6}H_{12}O_{6}$
6+12+6 = 24 atoms in 1 molecule of glucose
$$(\frac{24 atoms}{\text{1 molecule glucose}})(\frac{6.022*10^{23.}\text{ molecules of glucose}}{\text{1 mol glucose}})(\frac{5.99*10^{-14.} mol}{}) = 8.66*10^{11}\text{ atoms in glucose}$$Work for Exam II Practice Questions:¶
(3) Find limiting reagent, then solve using least: 30.2.10./(3.*63.) = 3.15 g NO
(6) 44.6 mL of the HCl is required. (n{1} = n{2})
(10)
- A gas is made up of a vast number of particles, and these particles are in constant random motion.
- Particles in a gas are infinitely small; they occupy no volume.
- Particles in a gas move in straight lines except when they collide with other molecules or with walls of the container. Collisions with each other and with the walls of the container are elastic, so that the total kinetic energy of the particles is conserved.
- Particles in a gas interact with each other only when collisions occur.
- The average kinetic energy of the particles in a gas is proportional to the absolute temperature of the gas and does not depend on the identity of the gas.
(15) (a) not allowed because must be no higher than n – 1
(b) allowed
(c) allowed
(d) not allowed because m has to be between – and +
(17) Solve E = hc/λ for wavelength; 2.3 × 10–4 m ; microwave
Work for Exam III Practice Questions:¶
(1-7)


(10) Which of the following contains both ionic and covalent bonds in the same compound?
a. BaCO3
b. MgCl2
c. BaO
d. H2S
e. SO
ANS (a) The barium cation is ionically bound to the carbonate anion. The “internal” bonds of the carbonate ion (i.e., C to O) are covalent.
(Extra) If silver atoms follow a face-centered cubic unit cell pattern, what is the length of this unit cell if the atomic radius is 144.4 pm?
a. 288 pm
b. 179 pm
c. 408 pm
d. 555 pm
Solution:
Refer the notes for an image of fcc.
$$ a^{2} + b^{2} = c^{2} \text{, where }b = a \text{ and } c = 4r$$$$ a^{2} + a^{2} = 2a^{2} = (4r)^{2} = 16r^{2}$$$$ a = (8r^{2})^{1/2} = \sqrt{8}r = \sqrt{8}(144 pm) = 407 pm$$Work for Exam IV Practice Questions:¶
(2)

(3) The activation energy is lowered which means there is a larger fraction of molecules with sufficient KE to react.
(16) N2 is consumed at 3.15 × 10–5 mol L–1 s–1. ; H2 is consumed at 9.44 × 10–5 mol L–1 s–1 mol L–1.
(17) Rate = k [A]
(26) 2.8 × 10–8 M
(27) [H2] = [I2] = 0.016 M; [HI] = 0.018 M
Work for Final Practice Questions:¶
(8) (a) Copper serves as the cathode in Cu-Co and Cu-Zn cells. Cobalt serves as the anode in the Ag-Co and Cu-Co cells.
(b) Ag-Zn generates the highest potential, and Cu-Co generates the lowest potential.
(14)
19Ne → 1β+ + 19F +ν
(15)
$$ ^{224}_{88}{Ra} \rightarrow \hspace{0.2cm} ^{220}_{86}{Rn} +\hspace{0.2cm} ^{4}_{2}{He}$$(16)
$$ ^{249}_{97}{Bk} \rightarrow \hspace{0.2cm} ^{249}_{98}{\text{Cf}} + \hspace{0.2cm} ^{0}_{-1}{e} $$(17)
$$ ^{209}_{83}{Bi} \hspace{0.2cm}+\hspace{0.2cm} ^{58}_{26}{Fe} \rightarrow \hspace{0.2cm} ^{266}_{109}{\text{Mt}} + \hspace{0.2cm} ^{1}_{0}{n} $$(18)
$$ ^{208}_{82}{Pb} \hspace{0.2cm}+\hspace{0.2cm} ^{62}_{28}{Ni} \rightarrow \hspace{0.2cm} ^{289}_{110}{\text{Ds}} + \hspace{0.2cm} ^{1}_{0}{n} $$(19)
$$ ^{208}_{82}{Pb} \hspace{0.2cm}+\hspace{0.2cm} ^{70}_{30}{Zn} \rightarrow \hspace{0.2cm} ^{277}_{112}{\text{Mt}} + \hspace{0.2cm} ^{1}_{0}{n} $$(20)
$$ ^{208}_{82}{Pb} \hspace{0.2cm}+\hspace{0.2cm} ^{62}_{28}{Ni} \rightarrow \hspace{0.2cm} ^{289}_{110}{\text{Ds}} + \hspace{0.2cm} ^{1}_{0}{n} $$